Amylyx Pharmaceuticals Reports the First Patient Dosing with AMX0035 in P-III Trial for the Treatment of Progressive Supranuclear Palsy (PSP)
Shots:
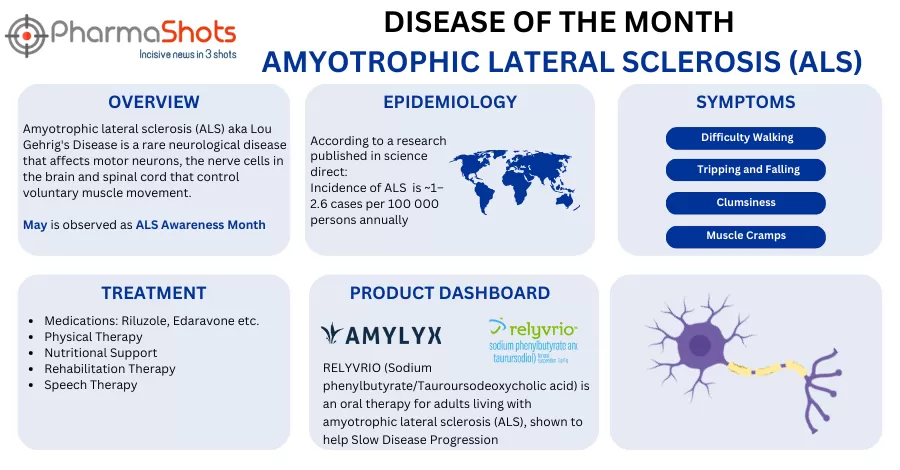
- The 1st patient was dosed in the P-III (AMX0035) trial evaluating the safety, efficacy & tolerability of taurursodiol in patients with progressive supranuclear palsy (PSP). The trial is expected to enroll 600 participants across 100 sites in areas incl. the US, Canada, the EU, UK & Japan
- The 1EP of the trial includes the change in disease progression from baseline at wk. 52 as measured by 28-item PSPRS whereas the 2EPs include disease progression as measured by 10-item PSPRS & motor aspects of activities of daily life measured by MDS-UPDRS Part II
- AMX0035 is a fixed-dose combination of sodium phenylbutyrate & taurursodiol. Moreover, it has been approved in the US for the treatment of amyotrophic lateral sclerosis (ALS) by the name Relyvrio
Ref: Amylyx | Image: Amylyx
Related News:- Amylyx Reports the Completion of Patient Enrollment in P-III Trial (PHOENIX) of AMX0035 for Amyotrophic Lateral Sclerosis
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release
Tags

Kritika is a content writer at PharmaShots. She is interested in covering recent innovations from the pharma & MedTech industry. She covers news related to Product approvals, clinical trial results, and updates. She can be contacted at connect@pharmashots.com.